 OCTOBER, 1889. Scientists flocked to Berlin for the annual meeting of the German Anatomical Society. The roster read like a who’s who of famous scientists of the day.
OCTOBER, 1889. Scientists flocked to Berlin for the annual meeting of the German Anatomical Society. The roster read like a who’s who of famous scientists of the day.
Into the fray marched a little-known Spaniard who’d spent years in Valencia and, later, Barcelona improving upon a method that made neurons visible under a microscope. Thanks to his patient tinkering, the Spaniard could see neurons in all their delicate, branching intricacy. He wanted to share his discoveries with other scientists. As he’d later say, he “gathered together for the purpose all my scanty savings and set out, full of hope, for the capital of the German Empire.”
In those days, scientific meetings were different from the parade of slideshows and posters sessions that they are today. The scientists at the 1889 meeting first read aloud from their papers and then took to their microscopes for demonstrations. The Spaniard unpacked his specimens and put them under several microscopes for the circulating scientists to view. Few came to see, in part because they expected little from a Spaniard. Spain was no scientific powerhouse. It lacked the scientific infrastructure and resources of countries like Germany, England, and France. What could one of its humble scientists possibly contribute to the meeting?
For the few curious gents who did stop by his demonstration, the Spaniard described his technique in broken French. Then he stepped aside and let them peer into the microscopes. Those who did became converts. The specimens spoke for themselves. Clear and complete, they revealed the intricate microarchitecture of neural structures like the retina and cerebellum.
Prominent German anatomists immediately adopted his technique and the Spaniard’s name quickly became known throughout the scientific community.
That name was Santiago Ramón y Cajal.
Ask any neuroscientist for his or her hero in the field and you are likely to hear this very name. Many consider him the founder of neurobiology as we know it today. The observations he made with his improved technique for seeing neurons allowed him to resolve a major controversy of the time and show that neurons are separate cells (as opposed to one huge, connected net). For his work, he won the Nobel Prize in Physiology or Medicine in 1906.
In short, he was an amazing guy who did amazing things – even though he wasn’t born in a wealthy nation known for science. Luckily, Cajal was able to get the tools and resources he needed to do his work. But what if he’d lived elsewhere, somewhere without the funds or equipment he needed? How far would that have set neuroscience back?
When I recently read an account of Cajal’s visit to Berlin, I found myself asking these questions. They reminded me of a Boston-based organization that is trying to equip the Cajals of today. The organization, a non-profit called Seeding Labs, partners with scientists, universities, and biomedical companies to equip stellar labs around the globe. (Full disclosure: The founder of Seeding Labs is the daughter of a family friend, which is how I first learned about the organization.)
The group’s core idea makes a lot of sense. Well-funded labs in the U.S. and other wealthy nations tend to update to newer models of their equipment often. These labs often discard perfectly functional older models that would be invaluable to scientists in developing nations. I’ve witnessed this kind of waste at major American universities. In the rush of doing science, people don’t have the time or energy to find new homes for their old autoclaves. They don’t even realize there’s a reason to try. While Seeding Labs now runs several programs to advance science in developing nations, its original aim was simply to turn one lab’s trash into another lab’s treasure.
I’m sure some struggling postdoc or assistant professor will read this post and scoff. Why devote energy to helping scientists in developing nations when we have a glut of scientists and a dearth of grants right here at home? It’s certainly true that research funding in America has tanked in recent years – a fact that needs to change. But in some countries the need is so great that a secondhand centrifuge could mean the difference between disappointment and discovery. That’s a pretty decent return on investment.
Here’s another benefit: labs in developing nations may be studying different problems than we are. They might focus on addressing local health or environmental concerns that we aren’t even aware of. So while scientists in wealthy nations find themselves racing to publish about well-trodden topics before competing labs, people in other countries may be researching crucial problems that wouldn’t otherwise be addressed.
And who knows? Perhaps these scientists are a good investment, in part, because of their relative isolation. Maybe a little distance from the scientific fray promotes ingenuity, creativity, and some good-old-fashioned tinkering. It certainly worked for Cajal.
____
Source: Stevens, Leonard A. Explorers of the Brain. Alfred A. Knopf, New York, 1971.
First photo credit: baigné par le soleil on Flickr, used via Creative Commons license
Second photo credit: Anonymous [Public domain], via Wikimedia Commons
 Humans learn about objects by exploring them. I
Humans learn about objects by exploring them. I 
 While we have five wonderful senses, humans rely most on our sense of sight. The allocation of real estate in the brain reflects this hegemony; a far greater chunk of your cerebral cortex is dedicated to vision than to any other sense. So when you encounter people, objects, and animals in the world, you typically use visual information to tell your lover from a toothbrush from your cat. And while it would be reasonable to expect your brain to process all of these items in the same way, it does nothing of the sort. Instead, the visual cortex segregates and plays favorites.
While we have five wonderful senses, humans rely most on our sense of sight. The allocation of real estate in the brain reflects this hegemony; a far greater chunk of your cerebral cortex is dedicated to vision than to any other sense. So when you encounter people, objects, and animals in the world, you typically use visual information to tell your lover from a toothbrush from your cat. And while it would be reasonable to expect your brain to process all of these items in the same way, it does nothing of the sort. Instead, the visual cortex segregates and plays favorites.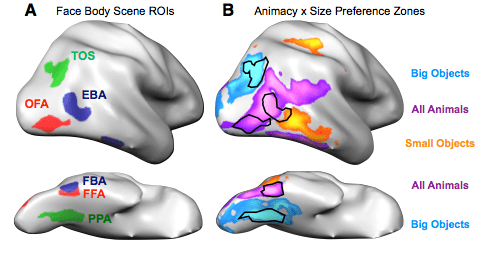
 More than a century ago, scientists discovered something usual about how people with
More than a century ago, scientists discovered something usual about how people with 